Search by topic:
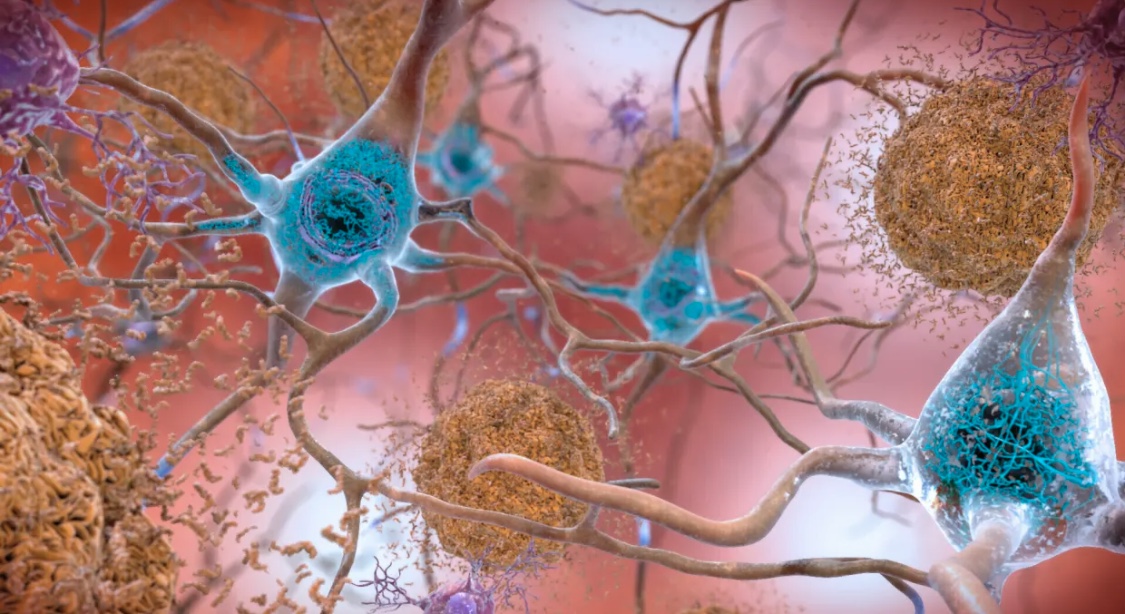
The drug, Leqembi, is the first to convincingly show it can slow the decline in memory and thinking that defines Alzheimer’s by targeting its underlying biology. The FDA approved it for patients with Alzheimer’s, specifically those with mild or early-stage disease.
Leqembi, from Japan’s Eisai and its US partner Biogen, is a rare success in a field accustomed to failed experimental treatments for the incurable condition. The delay in cognitive decline brought about by the drug likely amounts to just several months, but some experts say it could still meaningfully improve people’s lives.